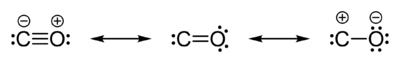Carbon monoxide
| Carbon monoxide | |
|---|---|
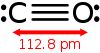 |
 |
|
Carbon monooxide
Carbon monoxide Carbon(II) oxide |
|
|
Other names
Carbonic oxide; Carbonyl
|
|
| Identifiers | |
| CAS number | 630-08-0 |
| PubChem | 281 |
| ChemSpider | 275 |
| EC number | 211-128-3 |
| UN number | 1016 |
| ChEBI | 17245 |
| RTECS number | FG3500000 |
|
SMILES
[O+]#[C-]
|
|
|
InChI
InChI=1/CO/c1-2
Key: UGFAIRIUMAVXCW-UHFFFAOYAT |
|
| Properties | |
| Molecular formula | CO |
| Molar mass | 28.010 g mol−1 |
| Appearance | colourless, odorless gas |
| Density | 0.789 g mL−1, liquid 1.250 g L−1 at 0 °C, 1 atm 1.145 g L−1 at 25 °C, 1 atm |
| Melting point |
−205 °C, 68 K, -337 °F |
| Boiling point |
−191.5 °C, 82 K, -313 °F |
| Solubility in water | 0.0026 g/100 mL (20 °C) |
| Solubility | soluble in chloroform, acetic acid, ethyl acetate, ethanol, ammonium hydroxide |
| Dipole moment | 0.112 D |
| Hazards | |
| MSDS | ICSC 0023 |
| EU Index | 006-001-00-2 |
| EU classification | Highly flammable (F+) Very toxic (T+) Repr. Cat. 1 |
| R-phrases | R61 R12 R26 R48/23 |
| S-phrases | S53 S45 |
| NFPA 704 |
 4
4
0
|
| Flash point | −191 °C |
| Autoignition temperature |
609 °C |
| Related compounds | |
| Related carbon oxides | Carbon dioxide Carbon suboxide Oxocarbons |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Carbon monoxide (CO), also called carbonic oxide, is a colorless, odorless and tasteless gas which is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal biological functions.
It consists of one carbon atom and one oxygen atom, connected by a covalent double bond and a dative covalent bond. It is the simplest oxocarbon, and is an anhydride of formic acid. In coordination complexes the carbon monoxide ligand is called carbonyl.
Carbon monoxide is produced from the partial oxidation of carbon-containing compounds; it forms when there is not enough oxygen to produce carbon dioxide (CO2), such as when operating a stove or an internal combustion engine in an enclosed space. Carbon monoxide burns with a blue flame, producing carbon dioxide.[1] Coal gas, which was widely used before the 1960s for domestic lighting, cooking and heating despite its toxicity, had carbon monoxide as a primary constituent. Some processes in modern technology, such as iron smelting, still produce carbon monoxide as a byproduct.[2]
Worldwide, the largest source of carbon monoxide is natural in origin, due to photochemical reactions in the troposphere which generate about 5 x 1012 kilograms per year.[3] Other natural sources of CO include volcanoes, forest fires, and other forms of combustion.
In biology, carbon monoxide is naturally produced by the action of heme oxygenase 1 and 2 on the heme from hemoglobin breakdown. This process produces a certain amount of carboxyhemoglobin in normal persons, even if they do not breathe any carbon monoxide. Following the first report that carbon monoxide is a normal neurotransmitter in 1993 [4], as well as one of three gases that naturally modulate inflammatory responses in the body (the other two being nitric oxide and hydrogen sulfide), carbon monoxide has received a great deal of clinical attention as a biological regulator. In many tissues, all three gases are known to act as anti-inflammatories, vasodilators and encouragers of neovascular growth.[5] Clinical trials of small amounts of carbon monoxide as a drug, are on-going.
Contents |
History
Carbon monoxide has been unknowingly used by humans since prehistoric times, for the smelting of iron and other metallic ores.[6] The gas was used for executions by the Greek and Romans in Classical Antiquity,[7] and was first described by the Spanish doctor Arnaldus de Villa Nova in the 11th century.[8] In 1776, the French chemist de Lassone produced CO by heating zinc oxide with coke, but mistakenly concluded that the gaseous product was hydrogen as it burned with a blue flame. The gas was identified as a compound containing carbon and oxygen by the Scottish chemist William Cumberland Cruikshank in the year 1800. Its toxic properties on dogs were thoroughly investigated by Claude Bernard around 1846.[9]
During World War II, carbon monoxide was used to keep motor vehicles running in parts of the world where gasoline was scarce. External charcoal or wood burners were fitted, and the carbon monoxide produced by gasification was piped to a carburetor. The carbon monoxide produced by this process is known as wood gas. Carbon monoxide was also reportedly used on a small scale during the Holocaust at some Nazi extermination camps, most notably by gas vans in Chelmno, and in the Action T4 "euthanasia" program.[10]
Molecular properties
Carbon monoxide has a molar mass of 28.0, which makes it slightly lighter than air whose average molar mass is 28.8.
The bond length between the carbon atom and the oxygen atom is 112.8 pm.[11] Atomic formal charge and electronegativity result in a small bond dipole moment with the negative end of the molecule on the carbon atom.[12] This is due to the highest occupied molecular orbital having energy much closer than that of carbon's p orbitals, despite oxygen's greater electronegativity. This means that greater electron density is found near the carbon atom. In addition, carbon's lower electronegativity creates a much more diffuse electron cloud, enhancing the polarizability. This is also the reason why almost all chemistry involving carbon monoxide occurs through the carbon atom, and not the oxygen.
The bond length of CO is consistent with a partial triple bond, and the molecule can be represented by three resonance structures:
In this model, the leftmost structure contributes the most. Carbon monoxide resembles molecular nitrogen (N2), and it has nearly the same molecular mass. The boiling point (82 K) and melting point (68 K) are very similar to those of N2 (77 K and 63 K respectively). The bond dissociation energy of 1072 kJ/mol is stronger than that of N2 (942 kJ/mol) and represents the strongest chemical bond known.[13]
The oxidation state of carbon in carbon monoxide is +2 in each of these structures. It is calculated by counting all the bonding electrons as belonging to oxygen, which is more electronegative than carbon. Only the two non-bonding electrons on carbon are assigned to carbon. In this count carbon then has only two valence electrons in the molecule compared to four in the free atom, so that its oxidation state is +2, and the molecule may be called carbon (II) oxide.
The ground electronic state of carbon monoxide is a singlet state.[14]
Biological and physiological properties
Toxicity

Carbon monoxide poisoning is the most common type of fatal air poisoning in many countries.[15] Carbon monoxide is colorless, odorless and tasteless, but highly toxic. It combines with hemoglobin to produce carboxyhemoglobin, which is ineffective for delivering oxygen to bodily tissues. This condition is known as anoxemia (citation needed). Concentrations as low as 667 ppm may cause up to 50% of the body's hemoglobin to convert to carboxyhemoglobin.[16] In the United States, the OSHA limits long-term workplace exposure levels above 50 ppm.[17]
The most common symptoms of carbon monoxide poisoning may resemble other types of poisonings and infections (such as the flu), including headache, nausea, vomiting, dizziness, fatigue and a feeling of weakness. Infants may be irritable and feed poorly. Neurological signs include confusion, disorientation, visual disturbance, syncope and seizures.[7]
Some descriptions of carbon monoxide poisoning include retinal hemorrhages, and an abnormal cherry-red blood hue[18]. In most clinical diagnoses these signs are seldom seen.[7]
Carbon monoxide binds to other molecules such as myoglobin and mitochondrial cytochrome oxidase. Exposures to carbon monoxide may cause significant damage to the heart and central nervous system, especially to the globus pallidus,[19] often with long-term sequelae. Carbon monoxide may have severe adverse effects on the fetus of a pregnant woman.[20]
Normal human physiology
Carbon monoxide is produced naturally by the human body as a signaling molecule. Thus, carbon monoxide may have a physiological role in the body, such as a neurotransmitter or a blood vessel relaxant.[21] Because of carbon monoxide's role in the body, abnormalities in its metabolism have been linked to a variety of diseases, including neurodegenerations, hypertension, heart failure, and inflammation.[21]
Microbiology
Carbon monoxide is a nutrient for methanogenic bacteria,[22] a building block for acetylcoenzyme A. This is the theme for the emerging field of bioorganometallic chemistry. In bacteria, carbon monoxide is produced via the reduction of carbon dioxide by the enzyme carbon monoxide dehydrogenase, an Fe-Ni-S-containing protein.[23]
CooA is a carbon monoxide sensor protein.[24] The scope of its biological role is still unknown; it may be part of a signaling pathway in bacteria and archaea. Its occurrence in mammals is not established.
Occurrence
Carbon monoxide occurs in various natural and artificial environments. Typical concentrations in parts per million are as follows:
| Concentration | Source |
|---|---|
| 0.1 ppm | Natural atmosphere level (MOPITT)[25] |
| 0.5 to 5 ppm | Average level in homes[26] |
| 5 to 15 ppm | Near properly adjusted gas stoves in homes[27] |
| 100 to 200 ppm | Exhaust from automobiles in the Mexico City central area[28] |
| 5,000 ppm | Exhaust from a home wood fire[29] |
| 7,000 ppm | Undiluted warm car exhaust without a catalytic converter[27] |
Atmospheric presence
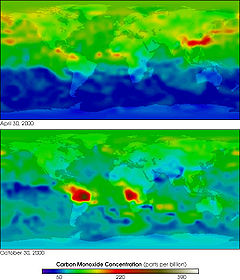
Carbon monoxide is present in small amounts in the atmosphere, chiefly as a product of volcanic activity but also from natural and man-made fires (such as forest and bushfires, burning of crop residues, and sugarcane fire-cleaning). The burning of fossil fuels also contributes to carbon monoxide production. Carbon monoxide occurs dissolved in molten volcanic rock at high pressures in the Earth's mantle.[30] Because natural sources of carbon monoxide are so variable from year to year, it is extremely difficult to accurately measure natural emissions of the gas.
Carbon monoxide has an indirect radiative forcing effect by elevating concentrations of methane and tropospheric ozone through chemical reactions with other atmospheric constituents (e.g., the hydroxyl radical, OH.) that would otherwise destroy them.[31] Through natural processes in the atmosphere, it is eventually oxidized to carbon dioxide. Carbon monoxide concentrations are both short-lived in the atmosphere and spatially variable.
Urban pollution
Carbon monoxide is a major atmospheric pollutant in some urban areas, chiefly from the exhaust of internal combustion engines (including vehicles, portable and back-up generators, lawn mowers, power washers, etc.), but also from improper burning of various other fuels (including wood, coal, charcoal, oil, paraffin, propane, natural gas, and trash).
Role in ground level Ozone levels
Carbon monoxide is part of the series of cycles of chemical reactions that form Photochemical smog. Along with aldehydes, it reacts photochemically to produce peroxy radicals. Peroxy radicals subsequently oxidize nitrogen oxide (NO) to nitrogen dioxide(NO).2.[32] Although this creation of NO2 is the critical step leading to low level ozone formation, it also increases this ozone in another, somewhat mutually exclusive way, by reducing the quantity of NO that is available to react with ozone.[32]
- Simplified, the net effect of the ozone cycle is:
- CO + 2O2 → CO2 + O3
Indoor pollution
In closed environments the concentration of carbon monoxide can easily rise to lethal levels. On average, 170 people in the United States die every year from carbon monoxide produced by non-automotive consumer products.[33] These products include malfunctioning fuel-burning appliances such as furnaces, ranges, water heaters and room heaters; engine-powered equipment such as portable generators; fireplaces; and charcoal that is burned in homes and other enclosed areas. The American Association of Poison Control Centers (AAPCC) reported 15,769 cases of carbon monoxide poisoning resulting in 39 deaths in 2007.[34] In 2005, the CPSC reported 94 generator-related carbon monoxide poisoning deaths.[33] Forty-seven of these deaths were known to have occurred during power outages due to severe weather, including Hurricane Katrina.[33] Still others die from carbon monoxide produced by non-consumer products, such as cars left running in attached garages. The Centers for Disease Control and Prevention estimates that several thousand people go to hospital emergency rooms every year to be treated for carbon monoxide poisoning.[35]
Carbon monoxide is also a minor constituent of tobacco smoke.
Production
Many methods have been developed for carbon monoxide's production.[36]
Laboratory preparation
Carbon monoxide is conveniently produced in the laboratory by the dehydration of formic acid, for example with sulfuric acid. Another method is heating an intimate mixture of powdered zinc metal and calcium carbonate, which releases CO and leaves behind zinc oxide and calcium oxide:
- Zn + CaCO3 → ZnO + CaO + CO
Industrial production
A major industrial source of CO is producer gas, a mixture containing mostly carbon monoxide and nitrogen, formed by combustion of carbon in air at high temperature when there is an excess of carbon. In an oven, air is passed through a bed of coke. The initially produced CO2 equilibrates with the remaining hot carbon to give CO. The reaction of O2 with carbon to give CO is described as the Boudouard equilibrium. Above 800 °C, CO is the predominant product:
- O2 + 2 C → 2 CO (ΔH = −221 kJ/mol)
Another source is "water gas", a mixture of hydrogen and carbon monoxide produced via the endothermic reaction of steam and carbon:
- H2O + C → H2 + CO (ΔH = +131 kJ/mol)
Other similar "synthesis gases" can be obtained from natural gas and other fuels.
Carbon monoxide is also is a byproduct of the reduction of metal oxide ores with carbon, shown in a simplified form as follows:
- MO + C → M + CO
Since CO is a gas, the reduction process can be driven by heating, exploiting the positive (favorable) entropy of reaction. The Ellingham diagram shows that CO formation is favored over CO2 in high temperatures.
Coordination chemistry

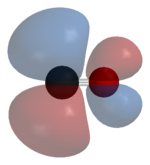
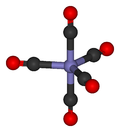
Most metals form coordination complexes containing covalently attached carbon monoxide. Only metals in lower oxidation states will complex with carbon monoxide ligands. This is because there must be sufficient electron density to facilitate back donation from the metal dxz-orbital, to the π*molecular orbital from CO. The lone pair on the carbon atom in CO, also donates electron density to the dx²−y² on the metal to form a sigma bond. In nickel carbonyl, Ni(CO)4 forms by the direct combination of carbon monoxide and nickel metal at room temperature. For this reason, nickel in any tubing or part must not come into prolonged contact with carbon monoxide (corrosion). Nickel carbonyl decomposes readily back to Ni and CO upon contact with hot surfaces, and this method was once used for the industrial purification of nickel in the Mond process.[37]
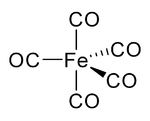
In nickel carbonyl and other carbonyls, the electron pair on the carbon interacts with the metal; the carbon monoxide donates the electron pair to the metal. In these situations, carbon monoxide is called the carbonyl ligand. One of the most important metal carbonyls is iron pentacarbonyl, Fe(CO)5:
Many metal-CO complexes are prepared by decarbonylation of organic solvents, not from CO. For instance, iridium trichloride and triphenylphosphine react in boiling 2-methoxyethanol or DMF) to afford IrCl(CO)(PPh3)2.
Organic and main group chemistry
In the presence of strong acids and water, carbon monoxide reacts with alkenes to form carboxylic acids in a process known as the Koch-Haaf reaction.[38] In the Gattermann-Koch reaction, arenes are converted to benzaldehyde derivatives in the presence of AlCl3 and HCl.[39] Organolithium compounds (e.g. butyl lithium) react with carbon monoxide, but these reactions have little scientific use.
Although CO reacts with carbocations and carbanions, it is relatively nonreactive toward organic compounds without the intervention of metal catalysts.[40]
With main group reagents, CO undergoes several noteworthy reactions. Chlorination of CO is the industrial route to the important compound phosgene. With borane CO forms an adduct, H3BCO, which is isoelectronic with the acylium cation [H3CCO]+. CO reacts with sodium to give products resulting from C-C coupling such as sodium acetylenediolate 2Na+·C2O2−2. It reacts with molten potassium to give a mixture of an organometallic compound, potassium acetylenediolate 2K+·C2O2−2, potassium benzenehexolate 6K+ C6O6−6,[41] and potassium rhodizonate 2K+·C6O2−6.[42]
The compounds cyclohexanehexone or triquinoyl (C6O6) and cyclopentanepentone or leuconic acid (C5O5), which so far have been obtained only in trace amounts, can be regarded as polymers of carbon monoxide.
At pressures of over 5 gigapascals, carbon monoxide disproportionates into carbon dioxide (CO2) and is a solid polymer of carbon and oxygen, in 3:2 atomic ratio.[43][44]
Uses
Chemical industry
Carbon monoxide is an industrial gas that has many applications in bulk chemicals manufacturing.[45]
Large quantities of aldehydes are produced by the hydroformylation reaction of alkenes, carbon monoxide, and H2. Hydroformylation is coupled to the Shell Higher Olefin Process to give precursors to detergents. Methanol is produced by the hydrogenation of carbon monoxide. In a related reaction, the hydrogenation of carbon monoxide is coupled to C-C bond formation, as in the Fischer-Tropsch process where carbon monoxide is hydrogenated to liquid hydrocarbon fuels. This technology allows coal or biomass to be converted to diesel.
In the Monsanto process, carbon monoxide and methanol react in the presence of a homogeneous rhodium catalyst and hydroiodic acid to give acetic acid. This process is responsible for most of the industrial production of acetic acid.
An industrial scale use for pure carbon monoxide is purifying nickel in the Mond process.
Meat coloring
Carbon monoxide is used in modified atmosphere packaging systems in the US, mainly with fresh meat products such as beef, pork, and fish to keep them looking fresh. The carbon monoxide combines with myoglobin to form carboxymyoglobin, a bright cherry red pigment. Carboxymyoglobin is more stable than the oxygenated form of myoglobin, oxymyoglobin, which can become oxidized to the brown pigment, metmyoglobin. This stable red color can persist much longer than in normally packaged meat.[46] Typical levels of carbon monoxide used in the facilities that use this process are between 0.4% to 0.5%.
The technology was first given "generally recognized as safe" (GRAS) status by the U.S. Food and Drug Administration (FDA) in 2002 for use as a secondary packaging system, and does not require labeling. In 2004 the FDA approved CO as primary packaging method, declaring that CO does not mask spoilage odor.[47] Despite this ruling, the process remains controversial for fears that it masks spoilage.[48] In 2007 a bill[49] was introduced to the United States House of Representatives to label modified atmosphere carbon monoxide packaging as a color additive, but the bill died in subcommittee. The process is banned in many other countries, including Canada, Japan, Singapore and the European Union.[50][51][52]
Medicine
In biology, carbon monoxide is naturally produced by the action of heme oxygenase 1 and 2 on the heme from hemoglobin breakdown. This process produces a certain amount of carboxyhemoglobin in normal persons, even if they do not breathe any carbon monoxide.
Following the first report that carbon monoxide is a normal neurotransmitter in 1993 [4], as well as one of three gasses which naturally modulate inflammatory responses in the body (the other two being nitric oxide and hydrogen sulfide), carbon monoxide has received a great deal of clinical attention as a biological regulator. In many tissues, all three gases are known to act as anti-inflammatories, vasodilators and encouragers of neovascular growth.[5] However, the issues are complex, as neovascular growth is not always beneficial, since it plays a role in tumor growth, and also the damage from wet macular degeneration, a disease for which smoking (a major source of carbon monoxide in the blood, several times more than natural production) increases the risk from 4 to 6 times.
Studies involving carbon monoxide have been conducted in many laboratories throughout the world for its anti-inflammatory and cytoprotective properties. These properties could potentially be used to prevent the development of a series of pathological conditions including ischemia reperfusion injury, transplant rejection, atherosclerosis, severe sepsis, severe malaria or autoimmunity. Clinical tests involving humans have been performed, however the results have not yet been released.[53]
See also
- Carbon monoxide (data page)
- Carbon monoxide poisoning
- Boudouard reaction
- Criteria air contaminants
- List of highly toxic gases
- Undersea and Hyperbaric Medical Society – hyperbaric treatment for CO poisoning
- Carbon monoxide detector
- Rubicon Foundation research articles on CO Poisoning
- Molecular cloud
References
- ↑ Carbon Monoxide - Molecule of the Month, Dr Mike Thompson, Winchester College, UK
- ↑ Robert U. Ayres, Edward H. Ayres (2009). Crossing the Energy Divide: Moving from Fossil Fuel Dependence to a Clean-Energy Future. Wharton School Publishing. p. 36. ISBN 0137015445. http://books.google.com/?id=KQqLicqcTM8C&pg=PA36.
- ↑ Weinstock, B.; Niki, H. (1972). "Carbon Monoxide Balance in Nature". Science 176 (32): 290. doi:10.1126/science.176.4032.290. PMID 5019781.
- ↑ 4.0 4.1 New York Times article. Accessed May 2, 2010
- ↑ 5.0 5.1 Li, L; Hsu, A; Moore, PK (2009). "Actions and interactions of nitric oxide, carbon monoxide and hydrogen sulphide in the cardiovascular system and in inflammation--a tale of three gases!". Pharmacology & therapeutics 123 (3): 386–400. doi:10.1016/j.pharmthera.2009.05.005. PMID 19486912.
- ↑ William L. Roberts (1983). Hot rolling of steel. CRC Press. p. 4. ISBN 0824713451. http://books.google.com/?id=pqt_DwrJcXIC&pg=PA4.
- ↑ 7.0 7.1 7.2 Blumenthal, Ivan (1 June 2001). "Carbon monoxide poisoning". J R Soc Med (The Royal Society of Medicine) 94 (6): 270–272. PMID 11387414. PMC 1281520. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=11387414. Retrieved May 2009.
- ↑ McVaugh, Michael (1970). "Arnald of Villanova". Dictionary of Scientific Biography. 1. New York: Charles Scribner's Sons. pp. 289–291. ISBN 0684101149.
- ↑ Rosemary H. Waring, Glyn B. Steventon, Steve C. Mitchell (2007). Molecules of death. Imperial College Press. p. 38. ISBN 1860948146. http://books.google.com/?id=FvgNKPxb43IC&pg=PA38.
- ↑ Martin Kitchen (2006). A history of modern Germany, 1800-2000. Wiley-Blackwell. p. 323. ISBN 1405100419. http://books.google.com/?id=_04R6DoPxLoC&pg=PT323.
- ↑ O. R. Gilliam, C. M. Johnson and W. Gordy (1950). "Microwave Spectroscopy in the Region from Two to Three Millimeters". Physical Review 78 (2): 140. doi:10.1103/PhysRev.78.140.
- ↑ W. Kutzelnigg (2002). Einführung in die Theoretische Chemie. Wiley-VCH. ISBN 3-527-30609-9.
- ↑ Common Bond Energies (D) and Bond Lengths (r)
- ↑ http://www.mpe.mpg.de/lab/CO/co.html Last accessed June 22, 2010.
- ↑ Omaye ST. (2002). "Metabolic modulation of carbon monoxide toxicity". Toxicology 180 (2): 139–150. doi:10.1016/S0300-483X(02)00387-6. PMID 12324190.
- ↑ Tikuisis, P; Kane, DM; McLellan, TM; Buick, F; Fairburn, SM (1992). "Rate of formation of carboxyhemoglobin in exercising humans exposed to carbon monoxide.". Journal of applied physiology (Bethesda, Md. : 1985) 72 (4): 1311–9. PMID 1592720.
- ↑ "OSHA CO guidlines". OSHA. http://www.osha.gov/SLTC/healthguidelines/carbonmonoxide/recognition.html. Retrieved May 2009.
- ↑ Ganong, William F (2005). "37". Review of medical physiology (22 ed.). McGraw-Hill. p. 684. ISBN 0071440402. http://books.google.com/?id=OLa8vDBXDD4C&dq=Ganong+WF.+Review+of+Medical+Physiology.+Norwalk+Ct:+Appleton+%26+Lange,+1995&printsec=frontcover. Retrieved May 2009.
- ↑ Prockop LD, Chichkova RI (2007). "Carbon monoxide intoxication: an updated review". J Neurol Sci 262 (1-2): 122–130. doi:10.1016/j.jns.2007.06.037. PMID 17720201.
- ↑ Susan Tucker Blackburn (2007). Maternal, fetal, & neonatal physiology: a clinical perspective. Elsevier Health Sciences. p. 325. ISBN 1416029443. http://books.google.com/?id=2y6zOSQcn14C&pg=PA325.
- ↑ 21.0 21.1 Wu, L; Wang, R (December 2005). "Carbon Monoxide: Endogenous Production, Physiological Functions, and Pharmacological Applications". Pharmacol Rev 57 (4): 585–630. doi:10.1124/pr.57.4.3. PMID 16382109. http://pharmrev.aspetjournals.org/cgi/content/full/57/4/585#XI._Conclusions_and_Perspectives. Retrieved May 26, 2009.
- ↑ R. K. Thauer (1998). "Biochemistry of methanogenesis: a tribute to Marjory Stephenson. 1998 Marjory Stephenson Prize Lecture" (Free). Microbiology 144 (9): 2377–2406. doi:10.1099/00221287-144-9-2377. PMID 9782487. http://mic.sgmjournals.org/cgi/reprint/144/9/2377.
- ↑ Jaouen, G., Ed. (2006). Bioorganometallics: Biomolecules, Labeling, Medicine. Weinheim: Wiley-VCH. ISBN 3-527-30990-X.
- ↑ Roberts, G. P.; Youn, H.; Kerby, R. L. (2004). "CO-Sensing Mechanisms". Microbiology and Molecular Biology Reviews 68 (3): 453–473. doi:10.1128/MMBR.68.3.453-473.2004. PMID 15353565.
- ↑ Committee on Medical and Biological Effects of Environmental Pollutants (1977). Carbon Monoxide. Washington, D.C.: National Academy of Sciences. pp. 29. ISBN 0-309-02631-8.
- ↑ Green W. "An Introduction to Indoor Air Quality: Carbon Monoxide (CO)". United States Environmental Protection Agency. http://www.epa.gov/iaq/co.html. Retrieved 2008-12-16.
- ↑ 27.0 27.1 Gosink, Tom (1983-01-28). "What Do Carbon Monoxide Levels Mean?". Alaska Science Forum. Geophysical Institute, University of Alaska Fairbanks. http://www.gi.alaska.edu/ScienceForum/ASF5/588.html. Retrieved 2007-12-01.
- ↑ Singer, Siegfried Fred. The Changing Global Environment. Dordrecht: D. Reidel Publishing Company. p. 90.
- ↑ Gosink T (January 28, 1983). "What Do Carbon Monoxide Levels Mean?". Alaska Science Forum. Geophysical Institute, University of Alaska Fairbanks. http://www.gi.alaska.edu/ScienceForum/ASF5/588.html. Retrieved December 16, 2008.
- ↑ Astrid Sigel, Roland K. O. Sigel (2009). Metal-Carbon Bonds in Enzymes and Cofactors. Royal Society of Chemistry. p. 243. ISBN 1847559158. http://books.google.com/?id=kcllhZcy53cC&pg=PA243.
- ↑ James Carrick White et al. (1989). Global climate change linkages: acid rain, air quality, and stratospheric ozone. Springer. p. 106. ISBN 0444015159. http://books.google.com/?id=gnaNHeU4rgQC&pg=PA106.
- ↑ 32.0 32.1 Ozone and other photochemical oxidants. National Academies. 1977. p. 23. ISBN 0309025311. http://books.google.com/?id=QkErAAAAYAAJ&pg=PA23.
- ↑ 33.0 33.1 33.2 U.S Consumer Product Safety Commission, Carbon Monoxide Questions and Answers, accessed 2009-12-04
- ↑ American Association of Poison Control Centers 2007 Annual Report
- ↑ Centers for Disease Control and Prevention, National Environmental Public Health Tracking Network, Carbon Monoxide Poisoning, accessed 2009-12-04
- ↑ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 200. ISBN 0-12-352651-5.
- ↑ Mond L, Langer K, Quincke F (1890). "Action of carbon monoxide on nickel". Journal of the Chemical Society 57: 749–753. doi:10.1039/CT8905700749.
- ↑ Koch, H.; Haaf, W. (1973), "1-Adamantanecarboxylic Acid", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv5p0020; Coll. Vol. 5: 20
- ↑ G. H. Coleman, David Craig (1943), "p-Tolualdehyde", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv2p0583; Coll. Vol. 2: 583
- ↑ Chatani, N.; Murai, S. "Carbon Monoxide" in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. doi:10.1002/047084289
- ↑ Werner Büchner, E. Weiss (1964) Zur Kenntnis der sogenannten «Alkalicarbonyle» IV[1] Über die Reaktion von geschmolzenem Kalium mit Kohlenmonoxid. Helvetica Chimica Acta, Volume 47 Issue 6, Pages 1415–1423. doi:10.1002/hlca.19640470604
- ↑ George Fownes (1869). A Manual of elementary chemistry. H.C. Lea. p. 678. http://books.google.com/?id=WM8HAAAAIAAJ&pg=PA678.
- ↑ Katz, Allen I.; Schiferl, David; Mills, Robert L. (1984). "New phases and chemical reactions in solid carbon monoxide under pressure". The Journal of Physical Chemistry 88: 3176. doi:10.1021/j150659a007.
- ↑ Evans, W. J.; Lipp, M. J.; Yoo, C.-S.; Cynn, H.; Herberg, J. L.; Maxwell, R. S.; Nicol, M. F. (2006). "Pressure-Induced Polymerization of Carbon Monoxide: Disproportionation and Synthesis of an Energetic Lactonic Polymer". Chemistry of Materials 18: 2520. doi:10.1021/cm0524446.
- ↑ Elschenbroich, C.;Salzer, A. ”Organometallics : A Concise Introduction” (2nd Ed) Wiley-VCH: Weinheim, 2006. ISBN 3-527-28165-7
- ↑ Sorheim, S, Nissena, H, Nesbakken, T (1999). "The storage life of beef and pork packaged in an atmosphere with low carbon monoxide and high carbon dioxide". Journal of Meat Science 52 (2): 157–164. doi:10.1016/S0309-1740(98)00163-6.
- ↑ Eilert EJ (2005). "New packaging technologies for the 21st century". Journal of Meat Science 71 (1): 122–127. doi:10.1016/j.meatsci.2005.04.003.
- ↑ "Low-Oxygen Packaging with CO: A Study in Food Politics That Warrants Peer Review". http://www.foodsafetymagazine.com/article.asp?id=644&sub=sub1. Retrieved April 18, 2007.
- ↑ "Carbon Monoxide Treated Meat, Poultry, and Seafood Safe Handling, Labeling, and Consumer Protection Act (Introduced in House)". http://www.thomas.gov/cgi-bin/query/z?c110:H.R.3115:.
- ↑ "Proof in the Pink? Meat Treated to Give It Fresh Look". ABC News. November 14, 2007. http://abcnews.go.com/GMA/Consumer/Story?id=3863064&page=1. Retrieved May 27, 2009.
- ↑ Carbon Monoxide in Meat Packaging: Myths and Facts. American Meat Institute. 2008. http://www.meatami.com/ht/a/GetDocumentAction/i/40141. Retrieved May 2009.
- ↑ "CO in packaged meat". Carbon Monoxide Kills Campaign. http://www.carbonmonoxidekills.com/packages_meat.htm. Retrieved May 2009.
- ↑ Johnson, Carolyn Y. (October 16, 2009). "Poison gas may carry a medical benefit". The Boston Globe. http://www.boston.com/news/local/massachusetts/articles/2009/10/16/poison_gas_may_carry_a_medical_benefit/?page=full. Retrieved October 16, 2009.
External links
- International Chemical Safety Card 0023
- National Pollutant Inventory - Carbon Monoxide
- NIOSH Pocket Guide to Chemical Hazards
- CID 281 from PubChem
- External MSDS data sheet
- Carbon Monoxide Detector Placement
- Carbon Monoxide Kills Awareness Campaign Site
- Carbon Monoxide Purification Process
- Carbon Monoxide Hazards with Backpacking Stoves
- USFDA IMPORT BULLETIN 16B-95, May 1999
- FDA Agency Response Letter GRAS Notice No. GRN 000083
- Microscale Gas Chemistry Experiments with Carbon Monoxide
- Instant insight outlining the physiology of carbon monoxide from the Royal Society of Chemistry
- Pictures of CO Poisoning Radiology and Pathology Images from MedPix.
|
|||||||||||
|
||||||||||||||||||||||||||||||||